Sentec's LuMon Electrical Impedance Tomography System Gets FDA Clearance
- Posted on September 8, 2025
- By Google News
- 4 Views
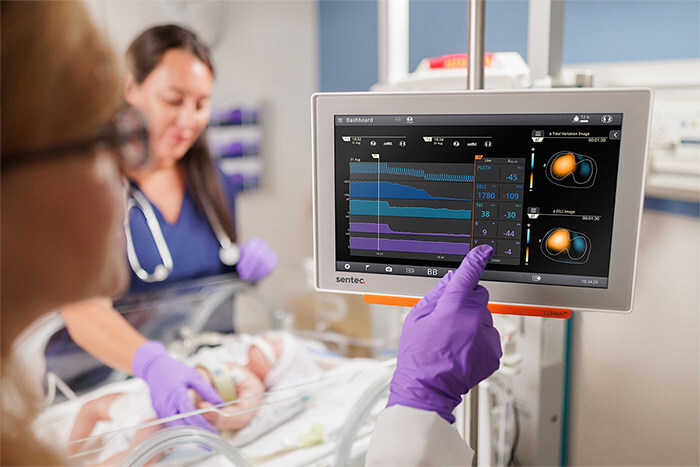
Sentec's LuMon Electrical Impedance Tomography System Gets FDA Clearance

Sept. 4, 2025 — Sentec recently announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its LuMon Electrical Impedance Tomography (EIT) system for premature infant, infant, adolescent and adult patients — making it the first EIT technology in the United States available for premature infants and for spontaneously breathing patients.




